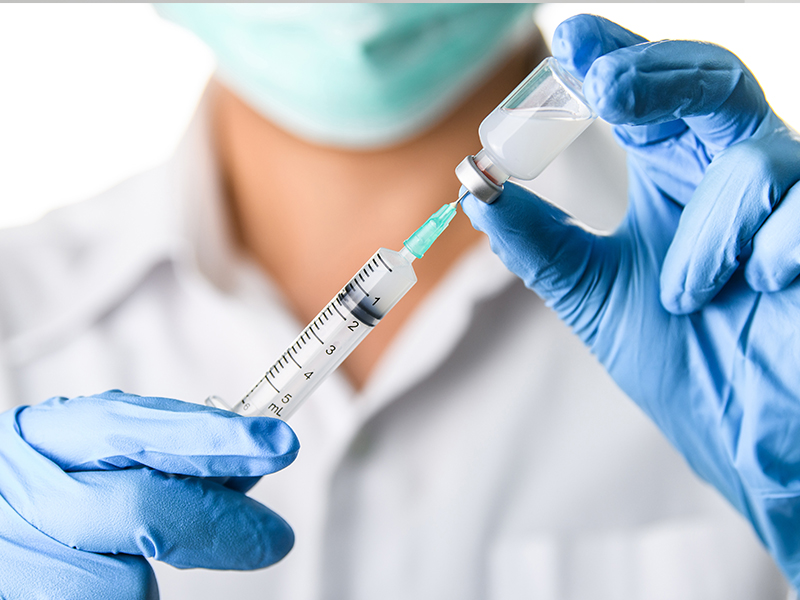
Jakarta: The phase 3 clinical trials of a potential covid-19 vaccine developed by Sinovac and Bio Farma run smoothly, Minister of Foreign Affairs Retno Marsudi has said.
“The preparation for timely vaccine delivery continues to be carried out on a prudent basis,” the Minister was quoted by the Cabinet Secretariat's website as saying.
According to her, the report of Head of the Covid-19 Vaccine Clinical Trials Team Prof. Dr. Kusnandi shows the phase 3 clinical trials of the vaccine developed by Sinovac and Bio Farma run smoothly and generated good results.
To support the development of covid-19 vaccine in Indonesia, the Ministry of Foreign Affairs continues to monitor the cooperation between State Owned Enterprise Bio Farma and Sinovac.
“On September 20-24, the Sinovac expert team conducted a visit to Bandung to review the Bio Farma vaccine production site and at the same time observe the implementation of phase 3 clinical trials carried out in Bandung area,” the Minister was quoted as saying.
Apart from the Sinovac vaccine, Bio Farma will also produce vaccine in collaboration with CEPI (Coalition for Epidemic Preparedness Innovations), and other vaccine candidates.
In the meantime, the Food and Drug Monitoring Agency (BPOM) will conduct an on-site visit to the Sinovac facility in Beijing to see firsthand the quality of the vaccine.
Moreover, the BPOM is also coordinating with Sinopharm and Group 42 (G42) as well as the United Arab Emirates (UEA) authorities regarding data sharing on the covid-19 vaccine clinical trials.
Cek Berita dan Artikel yang lain di Google News
FOLLOW US
Ikuti media sosial medcom.id dan dapatkan berbagai keuntungan